A special visit with a nonagenarian expert in pathogenic amoebae
Brain-eating amoeba brings together researchers from CZ Biohub SF, UCSF, and the California Department of Public Health
Treating diseases is much easier and more effective if you catch it early. But many conditions aren’t diagnosed until well after symptoms have surfaced, when, unfortunately, therapies are less effective. Scientists are beginning to understand that our own immune systems may be trained to detect the subtlest early warning signs of an illness long before we experience symptoms. Harnessing the potential of our immune cells—the only cells that come into contact with all of the tissues of our bodies—could lead to better outcomes for illnesses that are often diagnosed too late to be treated effectively, from ovarian and pancreatic cancer to Alzheimer’s and Parkinson’s.
Chan Zuckerberg Biohub New York brings together experts from Columbia University, The Rockefeller University, and Yale University to use a combination of cutting-edge experimental and computational technologies to genetically engineer immune cells to detect, prevent and even treat numerous diseases. Researchers will eavesdrop on immune cells’ conversations with other parts of the body, picking up on subtle clues that will eventually reveal organ-specific disease signals, thus helping doctors correct problems before they even manifest.
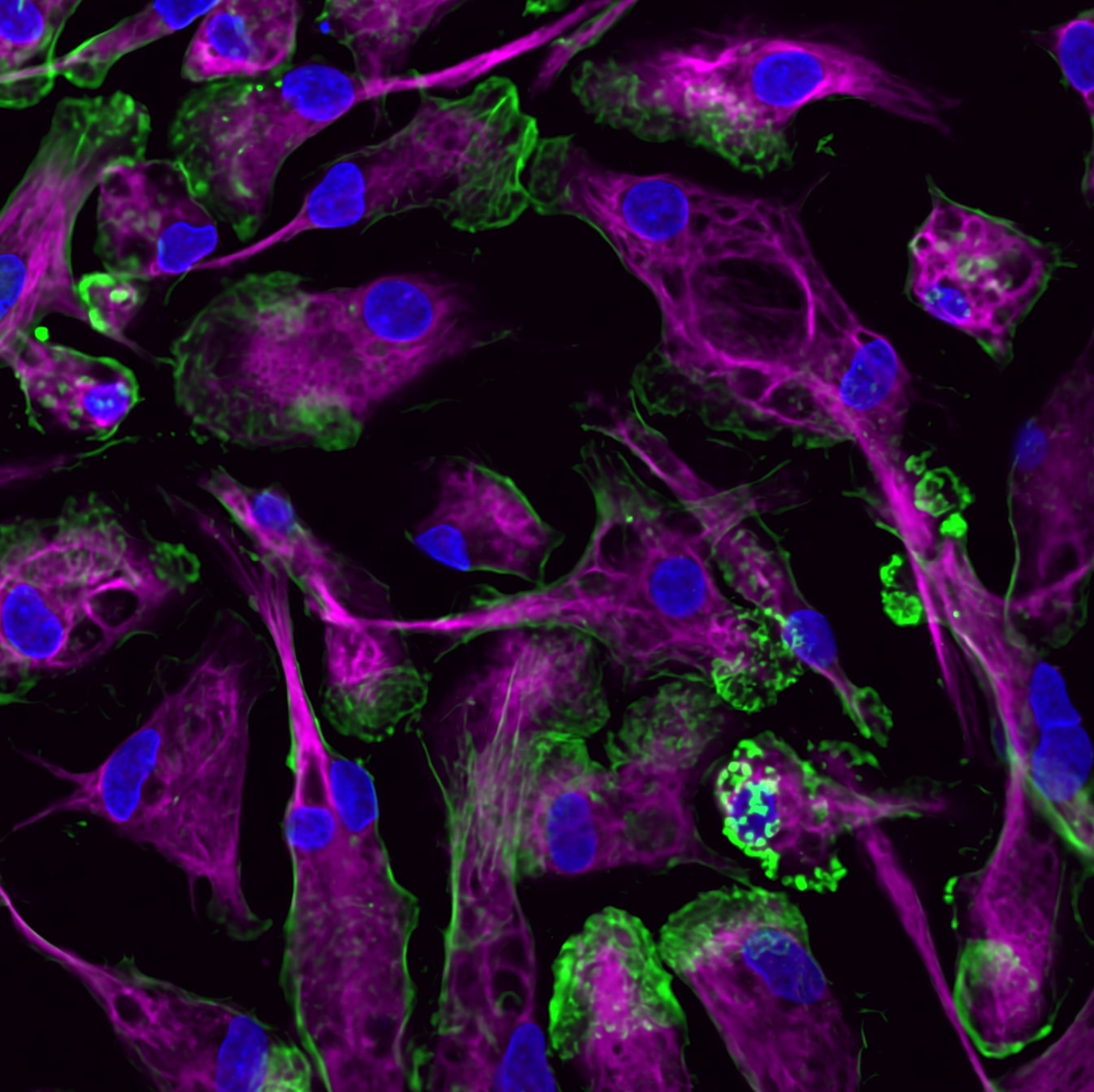
Such an approach could have radical implications in oncology. When cancer spreads to other places in the body (a process called metastasis), it becomes extremely challenging to treat. In fact, most cancer deaths are due to metastatic progression and not the growth of the primary tumor. Yet few drugs are effective or even designed to specifically prevent cancer’s spread.
Many unanswered questions remain about how cancer spreads, including why it happens in some patients but not others, and where it is most likely to metastasize to, such as the brain, lungs, or bones. But CZ Biohub NY Investigator and biomedical engineer Gordana Vunjak-Novaković of Columbia has a hunch that immune cells might hold the answers. Metastasis occurs when cancer cells break away from the original tumor and move to other parts of the body to settle in new areas. This process involves complex interactions between cancer cells and immune cells, she explains. At CZ Biohub NY, Vunjak-Novaković is developing innovative tools called “organ-on-a-chip” models to better understand this dialogue.
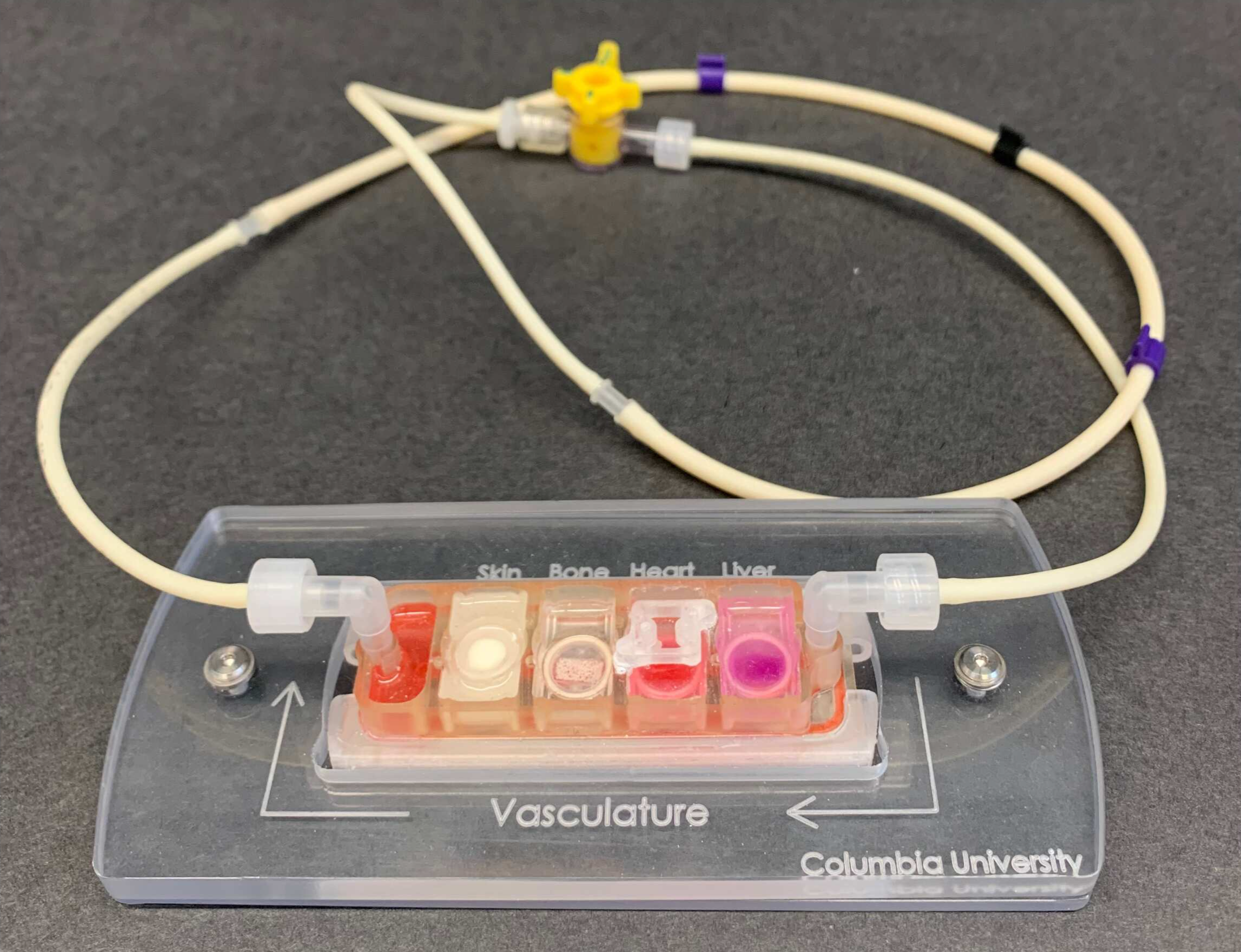
Organ-on-a-chip models are small, bioengineered devices that emulate the structure and function of multiple human organs, derived from the stem cells of the same individual, on a small bioreactor, including their ability to communicate by sharing cells and signals via circulating blood. These tools allow researchers to control and monitor the entire system and decipher both physiological and pathological interactions. In the context of cancer, organ-on–a-chip tools can recreate important features of a tumor’s environment, such as the chemical signals they send and receive to attract cells that will then prevent their detection by the immune system.
“We can study these human tissues individually, but with these devices you can also build the full physiological unit to understand why and how some patients develop metastasis and some don’t.”
Her models map the communication between bone marrow (where some immune cells are made) and tumors — with a specific focus on breast cancer. She hopes her experiments will provide insights into how the immune system responds to cancer and potentially influences its progression. But she also wants to tap into the highly individual nature of the disease.
“Some patients develop cancer quickly, some develop it more slowly,” says Vunjak-Novaković. “Some respond to treatment even if they’re at Stage 4, but most don’t.”
Animal models or cell lines aren’t well equipped to tell us why some cancers become particularly aggressive. By using primary human cells, organ-on-a-chip devices help model the human physiology more accurately, enabling scientists to study cellular cross-talk more accurately.
Vunjak-Novaković hopes that such tools could one day help clinicians predict whether a patient’s cancer might be more likely to spread, where it might metastasize to, and how best to treat it. Understanding the interactions between immune cells and cancer cells might also help scientists identify therapeutic targets for slowing or preventing metastasis, which could mean more effective cancer drugs in future.

It isn’t just cancer that researchers at CZ Biohub NY are interested in. A better understanding of the immune system could help us detect diseases like Parkinson’s and Alzheimer’s years before symptoms appear. CZ Biohub NY Investigator and immunobiologist Noah Palm, of Yale, focuses on illuminating interactions between the immune system and our gut bacteria in health and disease. He explores how certain gut microbes stimulate immune cells and what these interactions could teach us about different illnesses.
“We’re starting to understand that the immune system does more than just defend against infections,” says Palm. “It seems to also play a key role in maintaining the homeostatic balance of basically every organ and tissue.”
Determining how immune cells change and travel in the body, and what influence our gut bacteria have on these journeys, could one day help scientists create new therapies to detect and fix the earliest molecular signals of illness.
In his research, Palm adds or subtracts gut microbes in animal models to study their impact on health. So far, he’s found that certain microbes may lead to illnesses like inflammatory bowel disease. His work could have implications for preventing the condition — and other illnesses associated with immune overreaction — in susceptible individuals. “You can imagine that if you can prevent the microbe from doing what it’s doing, that might be sufficient to prevent development of the disease,” Palm sums up.
For his CZ Biohub NY project, Palm is pushing his research further. He believes there are still many immune-stimulating microbes in the human gut waiting to be discovered. These microbes might encourage immune cells to travel to other areas of the body, like the brain, where they could impact how diseases develop and serve as sentinels that report on incipient pathologies. For example, immune activity might be detectable in conditions like Parkinson’s long before symptoms appear. Palm’s goal is to figure out how specific microbes cause immune cells to change and move, and whether these cells can sense early tissue changes that ultimately lead to disease. Right now, treating Parkinson’s is difficult because it leads to a slow, gradual loss of irreplaceable brain cells that is often not detected until the damage is irreversible. But treatment could be more effective if doctors could detect the disease earlier, before those neurons start to die.
Uncovering the diagnostic potential of immune cells could pave the way for more personalized, precise treatments that intervene early, ultimately improving patient outcomes in a wide range of diseases. But such an ambitious mission requires long-term, strategic collaboration with multidisciplinary teams — one of the Chan Zuckerberg Biohub Network’s key strengths.
“This is incredibly complex research that none of our individual laboratories could carry out alone,” says Vunjak-Novaković. “To be effective, it is very important to have a critical mass of people aiming to reach the same goal, and that’s exactly what CZ Biohub NY is doing.”
Brain-eating amoeba brings together researchers from CZ Biohub SF, UCSF, and the California Department of Public Health
Learn More
A conversation with Steve Quake, Amy Herr, and the Stellar Science Foundation’s Takanori Takebe
Learn More
CZ Biohub San Francisco Investigator Hawa Racine Thiam’s new Stanford lab applies physics to the study of cells and their nuclei
Learn More
Stay up-to-date on the latest news, publications, competitions, and stories from CZ Biohub.
Cookies and JavaScript are required to access this form.