A simple model for an intricate, ever-evolving structure
Scientists use math and physics to address the mystery of just how the endoplasmic reticulum, an organelle essential to life at the cellular level, continually...
The telltale signs of inflammation are familiar to us all, such as red, painful swelling around a cut in the skin, fever and body aches during the flu, or coughing and shortness of breath from a chest cold.
Inflammation is the body’s natural, protective response to germs, injuries, and other irritants. However inflammation is not always beneficial. In many cases, it can be harmful, such as attacking the body’s own cells in rheumatoid arthritis and inflammatory bowel diseases like Crohn’s disease, illnesses that can last for years or a lifetime. Inflammation is so tightly intertwined with human health that an estimated 50% of all deaths are attributed to inflammation-related diseases, including cancer and heart disease.
Despite the importance of inflammation to human health, scientists and doctors have lacked a tool to accurately monitor the rise and fall of inflammation in the body. Such a technology seemed far off on the horizon—until now.
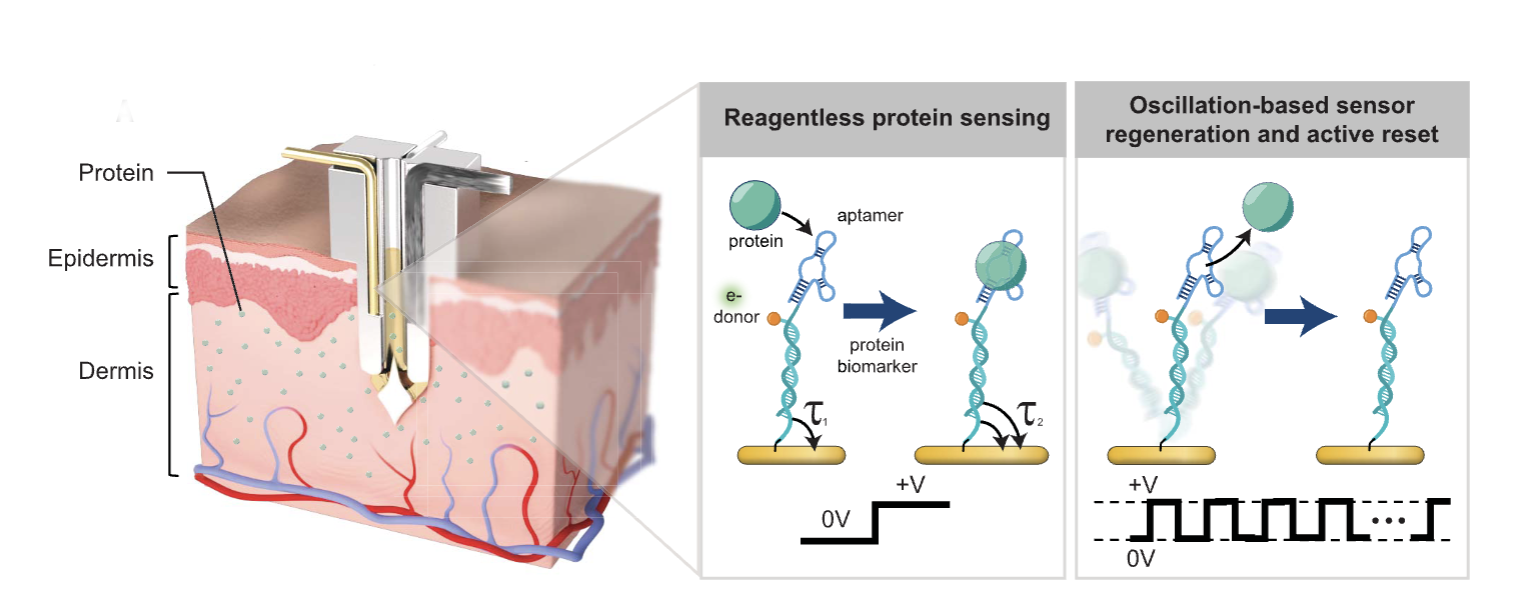
In a new paper in Science, bioengineers at the Chan Zuckerberg Biohub Chicago and Northwestern University unveil novel sensors that continuously measure concentrations of inflammatory proteins in living animals in real time. In an animal model of diabetes, the team showed that these “shaking” sensors, which catch and then fling target proteins away, accurately and sensitively detect changing levels of protein biomarkers of inflammation over time.
The work, completed in the first year after the Biohub’s formation, is a significant step toward understanding inflammation at a whole new level of resolution, and could enable breakthroughs in many diseases, from acute illnesses to chronic conditions. If researchers can more deeply understand and monitor inflammation in the human body in real-time, they may be able to intervene, and even prevent, inflammation-related diseases in the future.
That potential impact is comparable to how continuous glucose monitors have revolutionized the treatment of diabetes: improving patient outcomes, preventing disease complications, and significantly reducing costs across the healthcare system each year.
“This is a completely new capability—to be able to watch inflammation in real time,” says senior author Shana Kelley, president of CZ Biohub Chicago and professor of chemistry and bioengineering at Northwestern University. “There are a huge number of applications that we are now beginning to explore.”
When CZ Biohub Chicago’s work began in 2023, with a mission to develop new tools to understand and modulate inflammation, the team immediately set out to embed sensors into tissues to track inflammation.
Right away, they hit a roadblock. To track inflammation, the team first needed a way to measure levels of protein involved in inflammation. Most of today’s continuous monitoring devices measure small molecules like glucose, not proteins, which are larger and attach more tightly to receptors than small molecules.
Any sensor attempting to measure protein levels confronts a problem: proteins attach so strongly to target binding sites, such as those on antibodies, that they stick on for 20 hours or more. That strong attachment has made it near impossible to measure real-time fluctuations in protein levels in living animals over minutes, hours, or days, especially when levels decline.
To overcome that roadblock, the team had an idea. Kelley’s lab at Northwestern had previously developed protein sensors with a unique ability. The nanoscale sensors, dubbed “pendulum” sensors for their shape and swaying motion, move back and forth due to fluctuating electrical charges from an attached electrode. That movement produces enough force to shake off a bound protein, leaving the sensor empty and available to bind another, like a dog throw toy used to grab a ball from the ground, throw it, and then pick up a new ball.
Kelley’s lab had previously used the sensors to detect proteins in fluids such as saliva. Now, the CZ Chicago Biohub team wondered if those sensors might work in living animals to detect proteins in fluid just under the skin. Would the pendulum sensors be strong enough to shake off proteins in fluid inside a live animal, and could the engineers build a biocompatible device able to accurately record protein concentration changes?
It is rare for a scientific project to progress clearly and directly toward a goal, without the winding path of obstacles and redirections that are common to the scientific method. Yet to the delight of the scientists working on the project, the pendulum sensors worked exactly as hoped and planned.
“We wanted to figure out whether we could track inflammation,” says Kelley. “The first time we did it, it worked beautifully.”
The team deployed the sensors in rats used to study diabetes. Inflammation and diabetes are tightly linked: when diabetes is poorly controlled, it can cause inflammation and tissue damage, and it is one of the main reasons why people with diabetes experience complications, such as heart attacks, strokes and kidney problems.
The Biohub team engineered the sensors to detect two proteins that are markers of inflammation. They then built an implantable microdevice with the electrode and sensors inside a thin microneedle, the width of three human hairs, similar to microneedles inside continuous glucose monitors used today by people with diabetes to track blood glucose. They implanted the device on the skin of diabetic rats and initiated continuous monitoring of proteins in fluid just under the skin.
When the rats fasted, and blood glucose levels were under control, the researchers were able to observe the levels of inflammatory proteins drift down. Conversely, when the rats were injected with a substance to agitate the immune system, protein concentrations rapidly increased. The sensors were so sensitive, in fact, that each time a rat received a shot of insulin, the device detected the small, local spike in inflammation caused by the needle prick.
To confirm that the measurements from the sensors matched what was actually going on in the body, the team performed ELISA tests, a gold-standard laboratory method to detect proteins in bodily fluids, in blood and samples from fluid just under the skin. Following rigorous statistical testing of the sensor and ELISA measurements, the two sets of data agreed. The team also measured and confirmed that implanting the device did not itself cause widespread inflammation in the body.
Made of biocompatible and stable materials, the device and sensors are designed to operate safely in the body for at least two weeks, comparable to continuous glucose monitors, says Kelley.
Following the initial successful animal study, the team at the CZ Biohub Chicago is continuing to explore how inflammation changes as rats eat different foods and experience different environments and conditions. Bioengineers are also putting the sensors to work in the Biohub’s growing collection of instrumented tissues—tissue models of human organs embedded with thousands of sensors and sampling probes to monitor molecular and cellular signals with unprecedented resolution.
The ultimate goal, of course, is to find a way to use these tools for human health tracking. “Can we track inflammation in the human body on a continuous basis?” asks Kelley. Human participants wearing inflammation-tracking devices could help answer important questions about inflammation and health. For example, what happens to inflammation in the human body over the course of a day, a week, or a month? In which health conditions is inflammation a precursor or an effect of illness?
With that kind of information, wearable devices tracking protein levels could be applied to chronic disease management in any number of conditions, detecting dips or spikes in relevant protein concentrations even before a patient experiences symptoms.
“Like continuous glucose monitoring opened up the ability to monitor patients with diabetes, this has the same potential to allow us to monitor, and even prevent, many diseases,” says Kelley. “With inflammation, the sky’s the limit with what you could do for your health by keeping a handle on it.”
Scientists use math and physics to address the mystery of just how the endoplasmic reticulum, an organelle essential to life at the cellular level, continually...
Learn More
CZ Biohub San Francisco Investigator analyzes health record data to find evidence that a shingles vaccine can lower the chances of developing dementia
Learn More
Two Investigators share how their projects will leverage immune cells to detect cancer and neurodegenerative diseases sooner
Learn More
Stay up-to-date on the latest news, publications, competitions, and stories from CZ Biohub.
Marketing cookies are required to access this form.