Bioengineering
Developing custom instruments to accelerate the pace of discovery.
The Bioengineering team at CZ Biohub San Francisco designs and builds unique custom instruments that enable novel projects and accelerate the pace of discovery – from rugged, battery-powered portable devices for diagnosing an infectious disease in remote areas, to an automated multichannel protein purification device.
Projects
Fishpicker: Fish embryo & larvae picker/sorter
 Genetically modifying zebrafish to introduce desired fluorescence markers is an inefficient process, where only a small fraction of the fish properly express the markers. Many embryos go through the genetic modification protocol and the few embryos or larvae that express the markers correctly must be selected for growing and breeding. To make this process more efficient and less laborious, we have built a semi-automated instrument for retrieving/sorting individual zebrafish larvae and embryos. The device can image up to approximately 300 larvae or embryos on a custom array holder to check for the fluorescence markers, and automatically transfer select individual larvae/embryos from the array holder to individual wells on a multi-well plate. This is a collaboration with Adrian Jacobo’s Quantitative Tissue Morphogenesis team. Watch a video on the Fishpicker!
Genetically modifying zebrafish to introduce desired fluorescence markers is an inefficient process, where only a small fraction of the fish properly express the markers. Many embryos go through the genetic modification protocol and the few embryos or larvae that express the markers correctly must be selected for growing and breeding. To make this process more efficient and less laborious, we have built a semi-automated instrument for retrieving/sorting individual zebrafish larvae and embryos. The device can image up to approximately 300 larvae or embryos on a custom array holder to check for the fluorescence markers, and automatically transfer select individual larvae/embryos from the array holder to individual wells on a multi-well plate. This is a collaboration with Adrian Jacobo’s Quantitative Tissue Morphogenesis team. Watch a video on the Fishpicker!
FACS automation robot
 Sorting large numbers of cell samples to select the small fraction of cells in each sample that are expressing an engineered fluorescence marker is a very time-consuming and labor-intensive process. To make such work more efficient we built a system that automates sample transfer into and out of a commercial Sony FACS instrument (fluorescence-activated cell sorter). The automation standardizes gating, eliminating user-to-user variation in the created gates, reduces hands-on sorting labor by approximately 90%, and has the added benefit of reducing operator sample handling errors. This is a collaboration with Manuel Leonetti’s Intracellular Architecture team.
Sorting large numbers of cell samples to select the small fraction of cells in each sample that are expressing an engineered fluorescence marker is a very time-consuming and labor-intensive process. To make such work more efficient we built a system that automates sample transfer into and out of a commercial Sony FACS instrument (fluorescence-activated cell sorter). The automation standardizes gating, eliminating user-to-user variation in the created gates, reduces hands-on sorting labor by approximately 90%, and has the added benefit of reducing operator sample handling errors. This is a collaboration with Manuel Leonetti’s Intracellular Architecture team.
Automated protein purifier
 The purification of recombinant proteins is essential for both basic research and drug discovery and development. However, there are currently no good commercial instruments for purifying multiple proteins in parallel at milligram scales. To fill this gap we have built an automated, four-channel chromatography platform for parallelized protein purification at milligram scales. The device can purify up to four proteins (each with its own single column); has inputs for up to eight reagents, buffers, or solvents that can be directed to any of the four columns via a network of software-driven valves; and includes an automated fraction collector with 10 positions for 1.5 mL or 5.0 mL collection tubes, and four positions for 30 mL collection tubes, for each column output. This is a collaboration with John Pak’s Protein Science team.
The purification of recombinant proteins is essential for both basic research and drug discovery and development. However, there are currently no good commercial instruments for purifying multiple proteins in parallel at milligram scales. To fill this gap we have built an automated, four-channel chromatography platform for parallelized protein purification at milligram scales. The device can purify up to four proteins (each with its own single column); has inputs for up to eight reagents, buffers, or solvents that can be directed to any of the four columns via a network of software-driven valves; and includes an automated fraction collector with 10 positions for 1.5 mL or 5.0 mL collection tubes, and four positions for 30 mL collection tubes, for each column output. This is a collaboration with John Pak’s Protein Science team.
Ultrasensitive portable luminometer for serology studies in remote areas
 Although commercial benchtop luminometers exhibit exceptional low-light detection, handheld devices have lagged by orders of magnitude, making it difficult or impossible to transfer demanding luminescence assays from the lab into the field. We developed a compact, rugged, portable (battery powered) two-channel luminometer with performance on par with high-end benchtop instruments. By pairing robust and inexpensive silicon photomultiplier (SiPM) sensors with a low-profile shutter system, our design compensates for sensor non-idealities and thermal drift, achieving a limit of detection of 3E-20 moles of nanoluciferase, or approximately 1 fW of radiant optical power. Our devices were successfully deployed in a field study of SARS-CoV-2 antibody prevalence in Bangladesh, in collaboration with Cristina Tato’s Rapid Response team and Senjuti Saha of the CHRF, Dhaka, Bangladesh.
Although commercial benchtop luminometers exhibit exceptional low-light detection, handheld devices have lagged by orders of magnitude, making it difficult or impossible to transfer demanding luminescence assays from the lab into the field. We developed a compact, rugged, portable (battery powered) two-channel luminometer with performance on par with high-end benchtop instruments. By pairing robust and inexpensive silicon photomultiplier (SiPM) sensors with a low-profile shutter system, our design compensates for sensor non-idealities and thermal drift, achieving a limit of detection of 3E-20 moles of nanoluciferase, or approximately 1 fW of radiant optical power. Our devices were successfully deployed in a field study of SARS-CoV-2 antibody prevalence in Bangladesh, in collaboration with Cristina Tato’s Rapid Response team and Senjuti Saha of the CHRF, Dhaka, Bangladesh.
Remoscope: an automated label-free malaria detection instrument
 Malaria diagnosis remains a burden on global health care systems. Despite many new technologies, manual blood smear microscopy remains the gold standard for informing patient treatment, especially in endemic high transmission settings. Remoscope is a fully-automated imaging cytometer that uses machine learning to identify malaria in fresh whole blood without any need for fixation and staining, thus eliminating the reagents, labor, and variability associated with manual blood smear preparation and parasite counting. The instrument is currently being piloted on clinical cohorts in Uganda to evaluate diagnostic accuracy against current standards. The project is a collaboration with Joe DeRisi’s lab, the Dorsey and Rosenthal groups at UCSF, and the Infectious Disease Research Collaboration in Uganda.
Malaria diagnosis remains a burden on global health care systems. Despite many new technologies, manual blood smear microscopy remains the gold standard for informing patient treatment, especially in endemic high transmission settings. Remoscope is a fully-automated imaging cytometer that uses machine learning to identify malaria in fresh whole blood without any need for fixation and staining, thus eliminating the reagents, labor, and variability associated with manual blood smear preparation and parasite counting. The instrument is currently being piloted on clinical cohorts in Uganda to evaluate diagnostic accuracy against current standards. The project is a collaboration with Joe DeRisi’s lab, the Dorsey and Rosenthal groups at UCSF, and the Infectious Disease Research Collaboration in Uganda.
Automated cell culture passaging system
 Manually maintaining large numbers of cell libraries growing in multi-well plates is a very labor-intensive and error-prone process, especially when the initial cell density of the passaged cells must be tightly controlled. To address this issue, we have built a system to automatically passage cells cultured in multi-well plates, combining a commercial low-cost liquid handling robot, a custom-built microscopy-based cell counting device, and custom software. The system dissociates cells in the source plate, measures the resulting concentration in each well’s cell suspension, and transfers a predefined number of cells from each well in the source plate to one or more receiving plates. This automation reduces the hands-on time required to passage a plate by ~50 to ~70%. This is a collaboration with Manuel Leonetti’s Intracellular Architecture team.
Manually maintaining large numbers of cell libraries growing in multi-well plates is a very labor-intensive and error-prone process, especially when the initial cell density of the passaged cells must be tightly controlled. To address this issue, we have built a system to automatically passage cells cultured in multi-well plates, combining a commercial low-cost liquid handling robot, a custom-built microscopy-based cell counting device, and custom software. The system dissociates cells in the source plate, measures the resulting concentration in each well’s cell suspension, and transfers a predefined number of cells from each well in the source plate to one or more receiving plates. This automation reduces the hands-on time required to passage a plate by ~50 to ~70%. This is a collaboration with Manuel Leonetti’s Intracellular Architecture team.
Automated liquid exchange system for multi-well plate microscopy
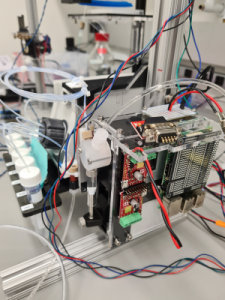 Many long imaging experiments on living cells in multi-well plates require regular media exchanges and/or stimulation with different reagents at different points in time. Doing this by hand is extremely laborious, and often impossible because of tight timing constraints when imaging fast responses of cells to chemical stimuli. To solve this bottleneck, we integrated a robotic system for automatically performing media exchanges and/or stimulation with up to five reagents or media into an existing confocal microscope. This is a collaboration with Manuel Leonetti’s Intracellular Architecture team.
Many long imaging experiments on living cells in multi-well plates require regular media exchanges and/or stimulation with different reagents at different points in time. Doing this by hand is extremely laborious, and often impossible because of tight timing constraints when imaging fast responses of cells to chemical stimuli. To solve this bottleneck, we integrated a robotic system for automatically performing media exchanges and/or stimulation with up to five reagents or media into an existing confocal microscope. This is a collaboration with Manuel Leonetti’s Intracellular Architecture team.
Organoid printer
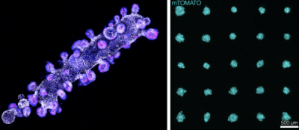 Current methods for creating organoids (small clusters of cells in culture that recapitulate some aspects of particular organs or tissues) result in extremely heterogeneous populations, both in terms of organoid size and composition. We have developed a system for automatically printing organoids of very well defined sizes and shapes with extremely high reproducibility. At the core of this instrument is a micro-pipette driven by a custom-designed piezo-driven extruder, with a dispensing resolution of 10pL, which allows for very precise dispensing of cell slurries and other reagents into gel matrices loaded into the wells of a multi-well plate. Cell slurries can be dispensed as the micro-pipette moves relative to the gel to print complex shapes and print arrays of those shapes in each well, all the while allowing real-time observation of the printing process via an integrated epi-fluorescence microscope. This is a collaboration with Zev Gartner’s lab at UCSF.
Current methods for creating organoids (small clusters of cells in culture that recapitulate some aspects of particular organs or tissues) result in extremely heterogeneous populations, both in terms of organoid size and composition. We have developed a system for automatically printing organoids of very well defined sizes and shapes with extremely high reproducibility. At the core of this instrument is a micro-pipette driven by a custom-designed piezo-driven extruder, with a dispensing resolution of 10pL, which allows for very precise dispensing of cell slurries and other reagents into gel matrices loaded into the wells of a multi-well plate. Cell slurries can be dispensed as the micro-pipette moves relative to the gel to print complex shapes and print arrays of those shapes in each well, all the while allowing real-time observation of the printing process via an integrated epi-fluorescence microscope. This is a collaboration with Zev Gartner’s lab at UCSF.
Sample holding systems for light sheet microscope
 For the ongoing Zebrahub project, we are developing systems to hold zebrafish embryos in novel light-sheet microscopes being developed by Loïc Ryer’s Organismal Architecture team. These systems allow automated high-throughput imaging of multiple embryos with reduced manual intervention, while keeping optimal environmental conditions for embryo development.
For the ongoing Zebrahub project, we are developing systems to hold zebrafish embryos in novel light-sheet microscopes being developed by Loïc Ryer’s Organismal Architecture team. These systems allow automated high-throughput imaging of multiple embryos with reduced manual intervention, while keeping optimal environmental conditions for embryo development.
Miscellaneous Hardware for CLIAhub
During the height of the pandemic the team designed and built multiple instruments for CLIAhub, a CLIA-certified COVID testing facility that the Biohub established at the height of the COVID-19 pandemic:
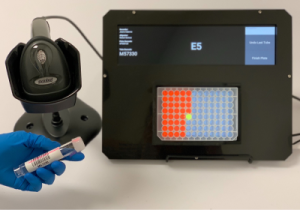 • Well-Lit: A device that helps eliminate errors when pipetting samples from barcoded tubes into multi-well plates, and when transferring samples from one multi-well plate to another. It has since become very useful for a variety of groups inside and outside of the Biohub (especially for the visiting scientists receiving training in metagenomic sequencing with the Rapid Response Team).
• Well-Lit: A device that helps eliminate errors when pipetting samples from barcoded tubes into multi-well plates, and when transferring samples from one multi-well plate to another. It has since become very useful for a variety of groups inside and outside of the Biohub (especially for the visiting scientists receiving training in metagenomic sequencing with the Rapid Response Team).
 • Bartender: A system that automatically fills large numbers of COVID-19 sample collection tubes with a preset volume of virus-preserving fluid. Two copies of the device were built for CLIAhub, and another two for the Stanford University COVID-19 testing lab.
• Bartender: A system that automatically fills large numbers of COVID-19 sample collection tubes with a preset volume of virus-preserving fluid. Two copies of the device were built for CLIAhub, and another two for the Stanford University COVID-19 testing lab.
 • Ventilator alarm: A small device that monitors the air pressure coming out of a hospital ventilator and generates audible alarms when it detects unsafe conditions for the patient, such as over-pressure, lack of breathing, etc. This device was designed to allow the use of cheap and easily available “transport ventilators” in the ICU when there was an acute shortage of ICU-rated ventilators (which have integrated alarms) in the early stages of the pandemic.
• Ventilator alarm: A small device that monitors the air pressure coming out of a hospital ventilator and generates audible alarms when it detects unsafe conditions for the patient, such as over-pressure, lack of breathing, etc. This device was designed to allow the use of cheap and easily available “transport ventilators” in the ICU when there was an acute shortage of ICU-rated ventilators (which have integrated alarms) in the early stages of the pandemic.


