Beating cardiomyocytes derived from human induced pluripotent stem cells (iPSC)
Cardiac Biointerface Engineering
Beating cardiomyocytes derived from human induced pluripotent stem cells (iPSC)
Our Research
Biointerface engineering to provide multiscale dynamic cardiac tissue models for disease modeling and monitoring
Cellular- and tissue-level biointerfaces are key structural elements for maintaining homeostasis and function in organs. We engineer cardiac biointerfaces—between cells, biomaterials, and devices —to develop advanced in vitro models for studying disease progression and therapeutic interventions.
The heart comprises many types of cells that maintain cardiac homeostasis and coordination. Local injury to the heart can disrupt communication and function among neighboring cells due to immune responses, leading to abnormal heartbeating, structural remodeling, and eventually, heart failure. Our goal is to develop tools and technologies to help us better understand the complex cellular and tissue interactions in the heart during disease progress.
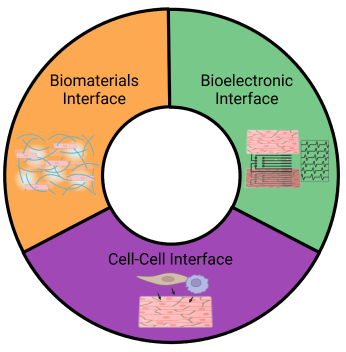
Three key biointerfaces to model and measure cardiac disease phenotype
3D Printed Multiscale Human Cardiac Tissue Model
Structural and functional recapitulation and multi-cellular environment are required to provide accurate in vitro models. We leverage 3D bioprinting techniques to build multiscale cardiac tissue models with both microscale and macroscale structural recapitulation. Our advanced cardiac tissue model will help us better understand disease mechanisms through spatiotemporal functional analysis.
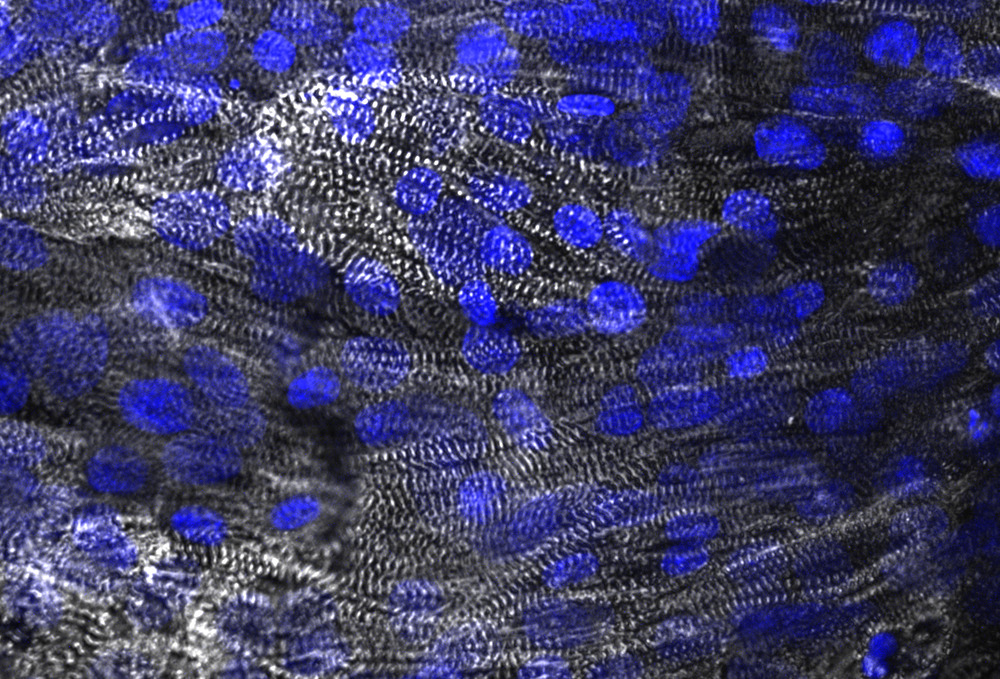
iPSC cardiomyocytes forming engineered cardiac tissues with structural alignment
Disease Biochemical Interfaces
The inflammation response involves a variety of biochemical and mechanical responses. Still, there is a lack of understanding of their spatial context within tissues. We aim to recreate the disease interface of cardiac local injury and structural remodeling in vitro, which provides a foundation for characterizing the interaction between biomolecule and cell responses.
Soft Bioelectronic Interface
Bioelectronic signals are an important key marker to inform their health status. We aim to provide a seamless soft bioelectronics interface that allows the functional monitoring of heart tissue without impeding functionality by mechanical disruption. Real-time monitoring will provide insight into spatiotemporal information that is lacking with time point-based analysis.


