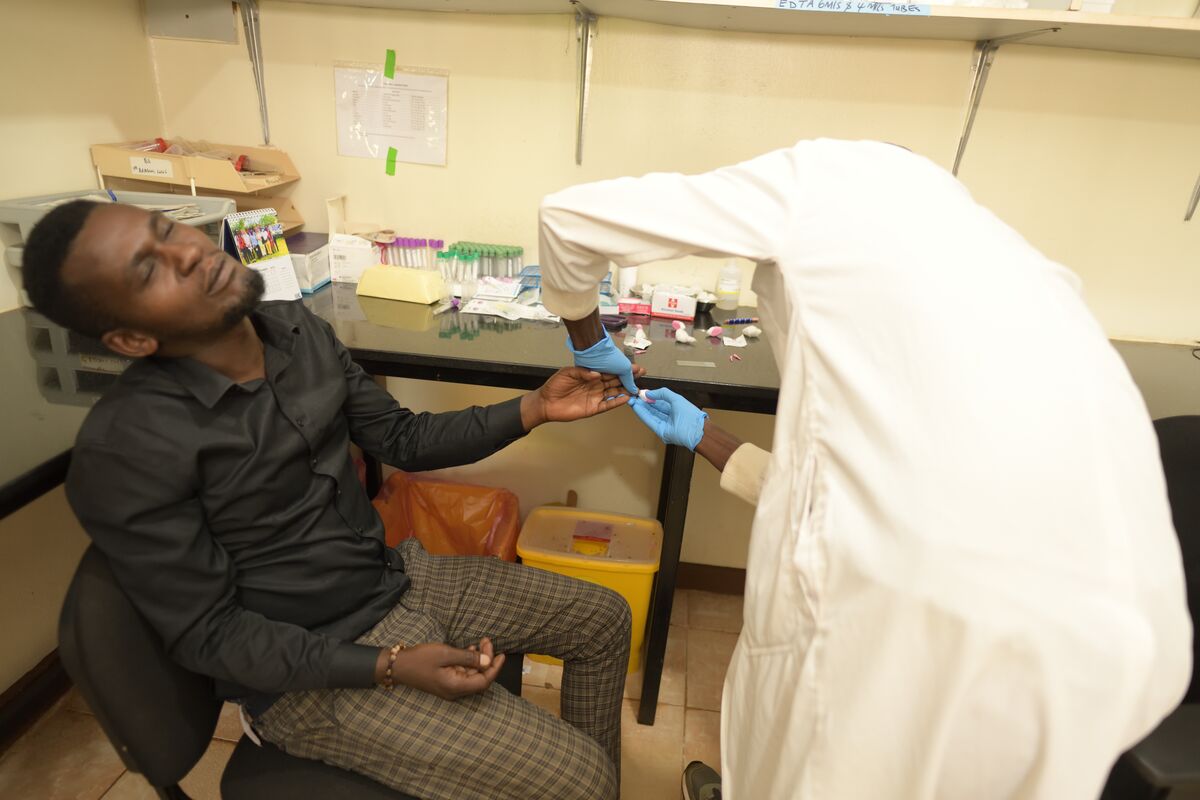
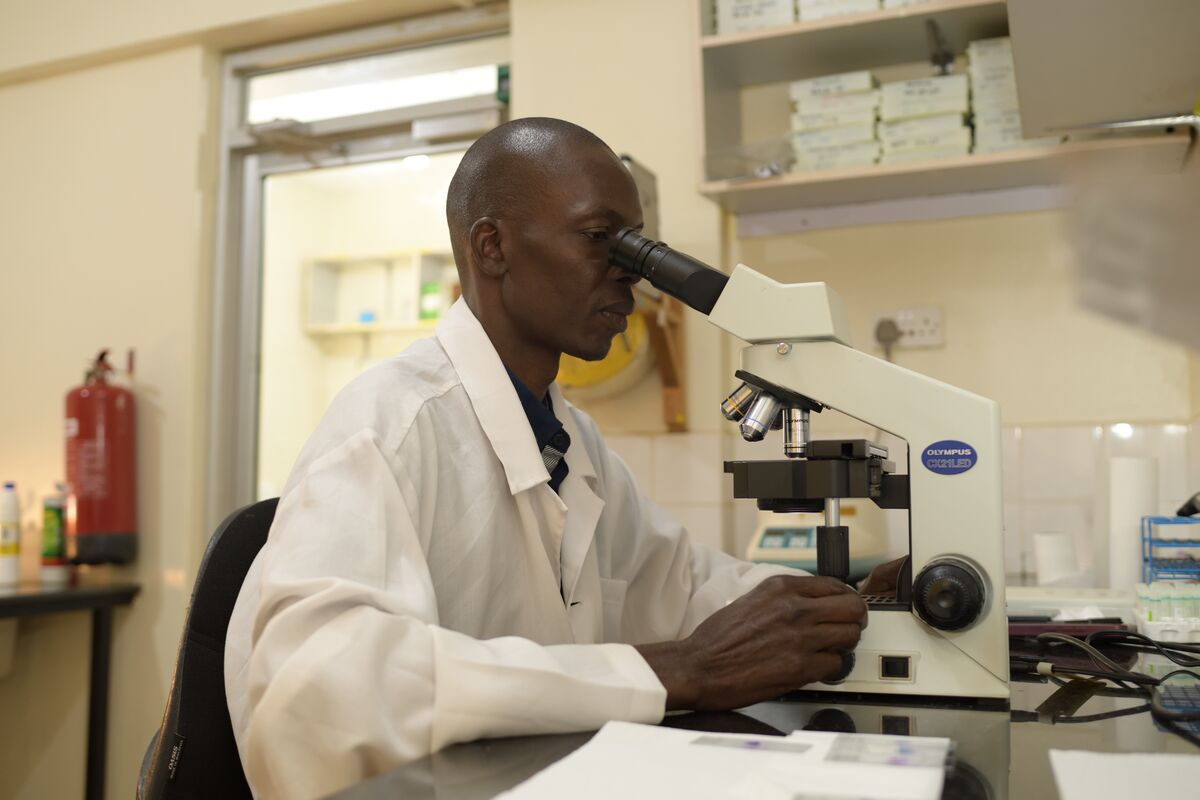
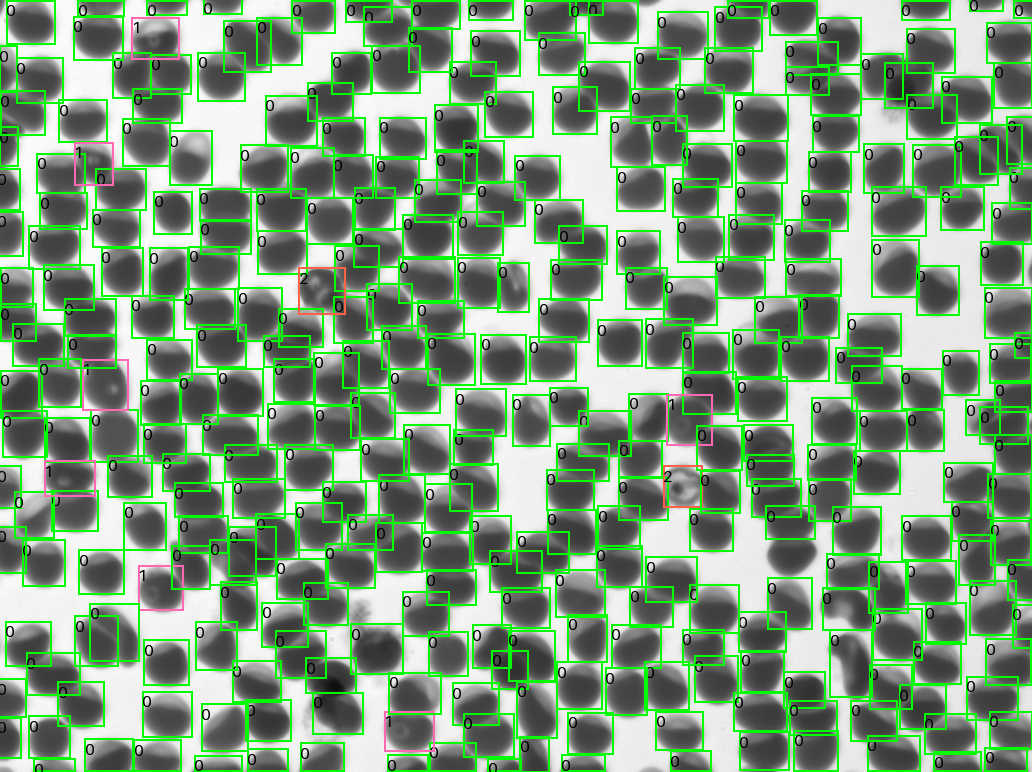
“We’ve been doing a lot of research in HIV, TB, and malaria,” says Olwoch, the IDRC lab manager for the past 12 years. “Over the years, we have tested a few automated microscopes with different functionalities. Most of them would use stained blood preparations that require the use of reagents that stain for a long period. And the turnaround time is a minimum of one hour. That’s time we can use for clinical management and treatment of the patient. It’s way too much.”
Olwoch was pleased not only by Remoscope’s speed but also that it could allow the clinic to eliminate the need for reagents, which can be expensive, and certain pieces of equipment.
What’s next
The Remoscope team is continuing to work with IDRC to evaluate testing with undiluted blood and other improvements that will lead them to scaling up. Separately, the team has struck up a collaboration with Goodlife Access, an independent California-based initiative working on healthcare issues in Rwanda, to study how the device performs in Rwanda. They will examine not only the diagnostic performance but also practical and economical outcomes.

A mother and child in Rwanda (Credit: Goodlife Access)
Beyond malaria, the Remoscope engineers are exploring additional applications for the device, which in the future could include detecting other bloodborne pathogens, genetic blood disorders, blood cancers such as leukemia, and more.
Meanwhile, the team is actively seeking partners who can help scale up the manufacture and distribution of the device, getting it to the locations where it’s most needed. “The most important thing for treating malaria is knowing who has the parasite, and who doesn’t, so accurate and fast diagnosis will help to quickly triage patients into appropriate treatment, instead of waiting potentially a day or more, when mosquitos can feed on that person and continue the cycle of infections,” says DeRisi. “Rapid treatment is required to break the chain.”