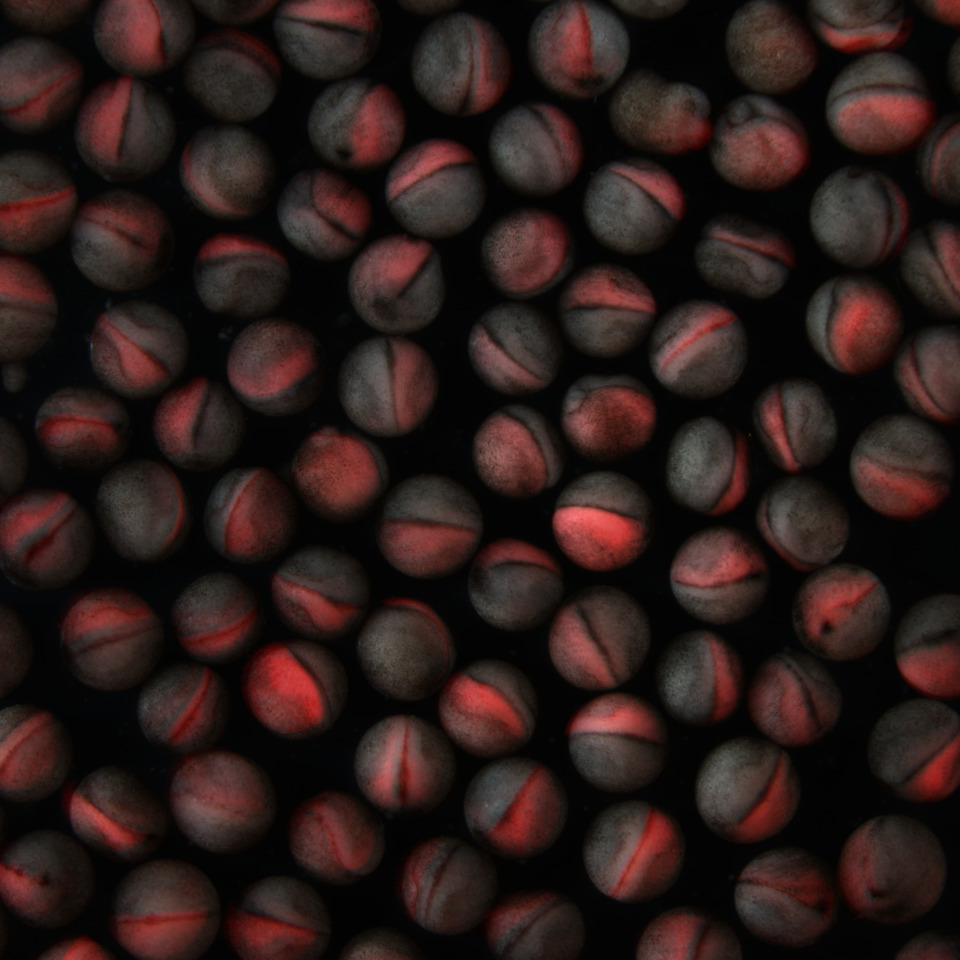
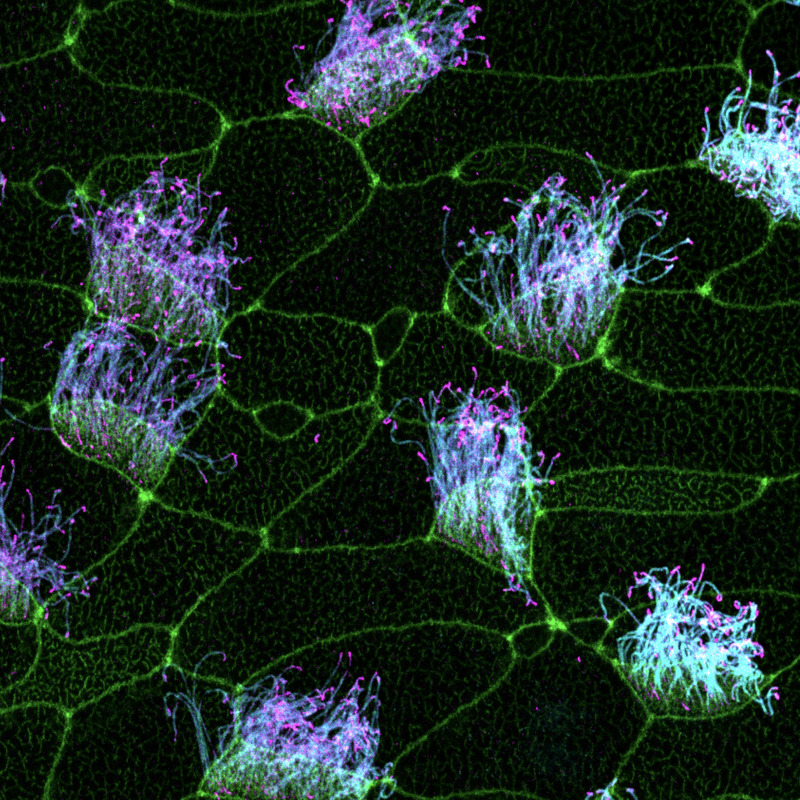
“This frog’s skin is just like a playground to understand ciliary biology,” Willsey says. “When you’re trying to understand where a gene has its impact and you’re looking at Xenopus, it’s very obvious if it happens to be in cilia.”
Using Xenopus embryos and human cells, Willsey and her colleagues have discovered that a number of autism-linked genes seem to carry the instructions for making proteins that build functional cilia. In a recent preprint that examined the expression and localization patterns of 30 proteins frequently linked to autism, Willsey and her team found that 12 of them could be found in both primary and motile cilia. When her team used genetic engineering to delete one of those genes, called SYNGAP1, in Xenopus embryonic cells, the mutation caused the corresponding SynGAP protein to travel away from the cilia, leading to defects in cilia structure.
Such work is setting the stage to help researchers understand how shifts in cellular biology might contribute to the core symptoms of profound autism, like trouble using language and socializing with others. But given the diverse roles that cilia play across our bodies, it may also help explain why conditions like gastrointestinal issues and cardiac problems so often occur alongside autism as well.
Other evidence supports these connections too. For example, people with Bardet-Biedl and Joubert syndromes — conditions known to be related to cilia defects — have an elevated risk of struggling with social challenges. And another recent Willsey lab preprint examining genes that carry risk for both autism and heart disease showed that a subset of these genes relate to cilia biology.
Cilia are also critical for our ability to sense our environment. They make up the specialized receptors in our eyes, ears, and noses, for example, that let us detect light, sound, and smell, respectively. This too could contribute to a condition like profound autism, in which individuals seem to struggle to navigate their environments.
“Our research is getting to the very heart of what drives autism and related health challenges, and cilia dysfunction looks to be a very important piece of the puzzle,” Willsey says. “This is the crescendo of my lab’s work so far.”