OUR RESEARCH
Human diseases, particularly those linked to inflammation, are complex in both space and time. To accurately understand inflammation, it is essential to make spatial and temporal measurements.
Spatial data provides a “map” of inflammation, while temporal data supplies the “timeline.” Together, spatiotemporal data creates a “movie” that illustrates the interactions, evolution, and reactions of biomolecules and cells throughout the course of inflammation. We believe this holistic perspective is crucial for deciphering inflammatory mechanisms and discovering new treatments.
Given the current technological landscape, no solution comprehensively addresses the needs of spatiotemporal analysis. With this challenge in mind, our interdisciplinary team is designing approaches to make spatiotemporal measurement and analysis of live tissues at the molecular level a reality.
RESEARCH PROJECTS

An array of hydrogel microneedles for spatiotemporal biomolecular sampling
The development of various omics technologies has enabled comprehensive analysis of both healthy and diseased tissues, facilitating the identification of specific disease biomarkers. However, the majority of these technologies are destructive and unsuitable for studying live tissues. Consequently, the dynamics of disease progression remain largely unexplored. We are developing a bioengineered platform that allows minimally invasive sampling of biomolecules at different locations and times within live tissues, thereby achieving spatiotemporal omics (STOM). We hope this new tool will help us better understand the initiation and early-stage progression of inflammation that becomes chronic.
Biofabrication of Tissue Mimics
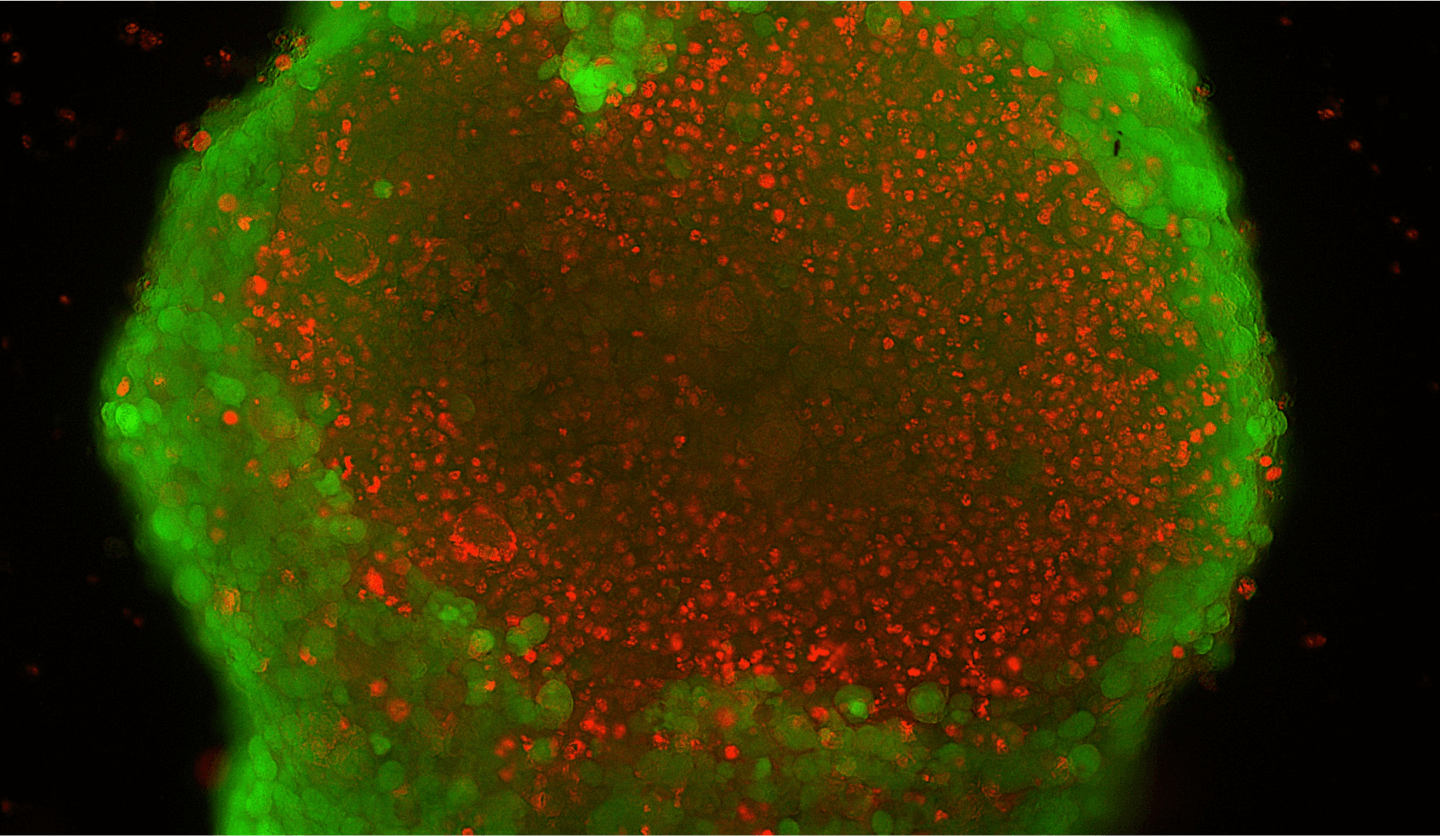
A fluorescent image showing the cellular morphology within large organoids
To test and validate our STOM platform, generating in vitro tissue mimics that accurately recapitulate in vivo tissue functions is crucial. However, the current methodologies for creating these tissue mimics are poorly defined. We are working on new concepts and designs to enhance the fabrication of tissue mimics by leveraging advanced biofabrication, bioassembly, and organ-on-chip techniques.
Immune Engineering of Inflammation
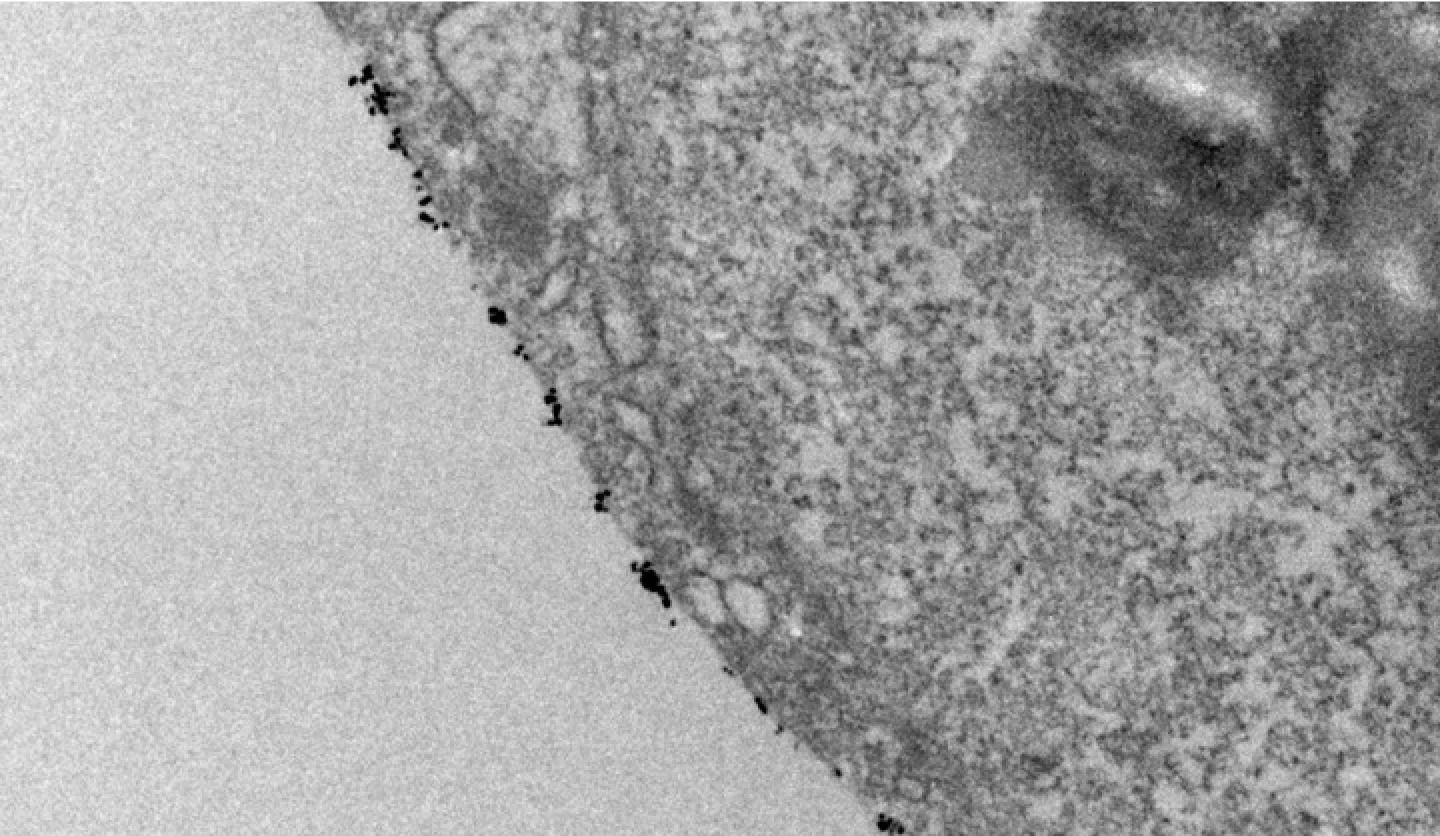
An electron microscopic picture of functionalized nanomaterials attached to the membrane of stem cells
It is well known that immune cells and immune systems play a central role in inflammation, yet the approaches to precisely modulate immune cells are limited, constraining our capability to manage undesired inflammation. We are designing new engineering techniques by combining nanotechnology, microfluidics, and material sciences to enhance our ability to target specific immune cell and inflammation-related cell populations.